how to draw 3d orbital molecules
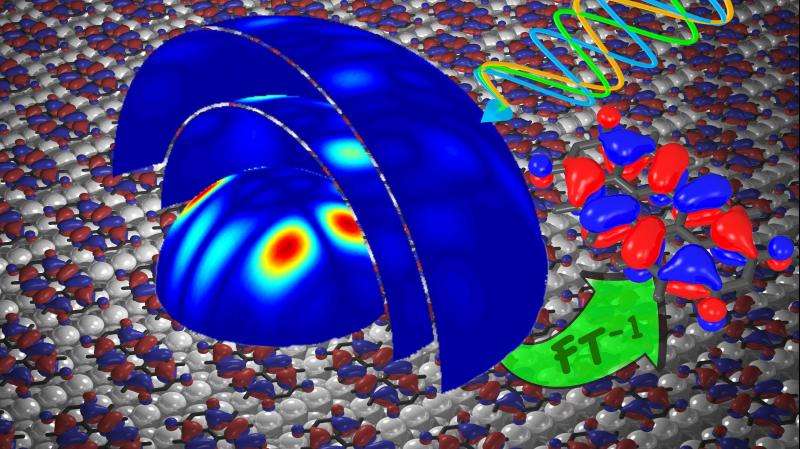
Many people volition call back them from physics lessons at school: Frequently represented equally colourful clouds or balloons, electron orbitals provide information on the whereabouts of the electrons in atoms and molecules. Scientists from the University of Graz, Forschungszentrum Jülich, and the Physikalisch-Technische Bundesanstalt take now succeeded in experimentally recording these structures in all 3 dimensions. They accomplished this by farther developing a method they had already practical two years ago to make these orbitals visible in two dimensions. Their findings accept now been published in the scientific journal Nature Communications.
In breakthrough physics, electrons conduct both as particles and as waves. The wave nature can be described by the spatial wave function, the orbital. "Orbitals contain information on the spatial distribution of the electrons at a certain energy. If they are known, all the relevant properties of a material tin exist derived," explains Prof. Peter Puschnig from the University of Graz. However, the laws of quantum mechanics prevent the directly observation of how an electron propagates equally a wave.
In 2004, a squad of Canadian and Japanese scientists used a high-energy laser to show that this orbital function can be imaged indirectly – at least for uncomplicated diatomic molecules. Nearly 10 years later, researchers from Graz and Jülich recorded orbitals of larger complex molecules for the showtime time, admitting simply in two dimensions. For their measurements, they used photoelectron spectroscopy, based on the photoelectric effect. In this process, a molecular layer on a silver surface is bombarded with photons (particles of light), causing the energetically excited electrons to be released. "The electrons do not merely wing effectually in space. Instead, their angular and energy distributions enable u.s. to draw conclusions about the molecular orbitals," says Puschnig.
By further refining this method, the scientists accept now succeeded in reconstructing the orbitals in all three dimensions. This meant that the experiment had to exist performed with various photon energies, i.e. different wavelengths of light, in the ultraviolet range. "Additional information on the 3rd dimension tin be obtained with variable wavelengths in much the same mode as a camera takes repeated pictures of ane object with a variable focus," explains Prof. Stefan Tautz from Forschungszentrum Jülich. However, it took a long fourth dimension earlier it was possible to combine the data gathered in different measurement series into one spatial model.
"Until now, we were unable to compare the measured intensities originating from dissimilar photon energies," says Prof. Michael Ramsey from the Department of Physics at the Academy of Graz. "Together with the photon free energy, the photon flux also changes, in other words, the absolute number of incoming photons which have to be known for the 3D reconstruction. Merely this number is difficult to measure precisely," adds Dr. Serguei Soubatch from Jülich's Peter Grünberg Institute (PGI-three).
In order to obtain comparable values, the Jülich researchers installed their detector at the Metrology Light Source (MLS) of the Physikalisch-Technische Bundesanstalt (PTB) in Berlin. "Our synchrotron radiations source is one of the few worldwide that provides a precisely calibrated photon flux," explains Dr. Alexander Gottwald from PTB. On the basis of the information from the calibrated measurements, the scientists at Graz were then able to reconstruct the electron distributions in three dimensions.
The research team from Jülich, Graz, and Berlin was thus able find the moving ridge function, which according to the rules of quantum mechanics is in fact considered unobservable. The results are long-sought proof of the orbital concept as such. In 1977, for case, the orbital theorist Kenichi Fukui, who together with Roald Hoffmann received the Nobel Prize for chemistry in 1981, described the concept of molecular orbitals equally having a "somewhat unreal nature" (Intern. J. Quantum Chem. 12, 277).
And as Hoffmann said in 1999, even theorists who use orbitals in their daily work practise non really credit them with the necessary reality: "[…] the physicists and chemists who use density functional theory and then fruitfully have more often than not shied away from attributing to […] orbitals the reality that (we call up) they deserve" (J. Am. Chem. Soc. 121, 3414).
The effect is also relevant for physics: "Our experiment provides important new concrete insights into the underlying photoelectric effect," says Stefan Tautz. Somewhat surprisingly, the electrons that are released can exist described in a manner very similar to free electrons – an thought that was rejected almost fifty years ago on the ground of the assumed scattering by the atomic cores.
More information: "Exploring three-dimensional orbital imaging with energy dependent photoemission tomography." Nature Communications (2015), DOI: 10.1038/NCOMMS9287
Citation: Researchers measure out electron orbitals of molecules in 3-D (2015, October 5) retrieved eight March 2022 from https://phys.org/news/2015-10-electron-orbitals-molecules-d.html
This document is subject area to copyright. Autonomously from whatever fair dealing for the purpose of private study or enquiry, no part may be reproduced without the written permission. The content is provided for information purposes only.
Source: https://phys.org/news/2015-10-electron-orbitals-molecules-d.html
0 Response to "how to draw 3d orbital molecules"
ارسال یک نظر